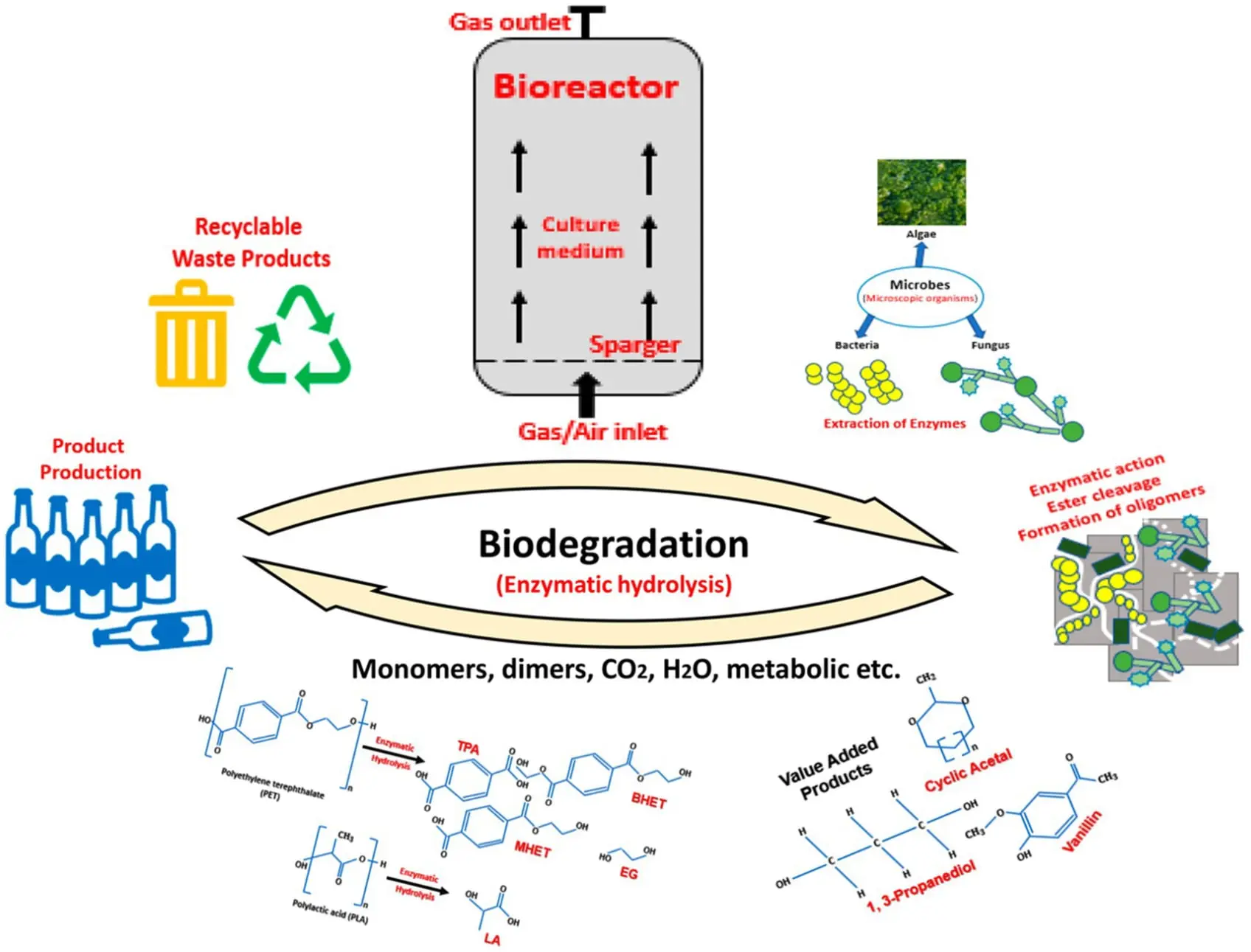
Polyethylene Terephthalate
Polyethylene terephthalate (PET), a significant polymer found in most consumer packaging, including cookie containers, soda bottles, fruit and salad packaging, and certain fibers and textiles, makes up 12% of all global waste.
PET-based plastics possess a high degree of crystallinity (30–50%), which is one of the principal reasons for their low rate of microbial degradation, which is projected to take more than 50 years for complete degraded in the natural environment, and hundreds of years if discarded into the oceans, due to their lower temperature and oxygen availability.
While polymer synthesis is an exothermic and relatively simple reaction dominated by the C–C bond formation, the opposite reaction, depolymerization, is an endothermic process. The energy-intensive characteristic of such processes makes it difficult to selectively break down the C–C bonds and achieve high yields of the desired products without also generating undesired radicals or highly reactive molecules that produce other unwanted products. Therefore, there is no universal or multi-purpose catalyst for all plastics, which means it is necessary to select the catalyst according to the polymers and the desired target of products needed.
*** Quantum Computing To The Rescue!!!