Quantum Computing To The Rescue
Ammonia is best known as a fertilizer, but could also be used as fuel, potentially making it one of the best decarbonization solutions.
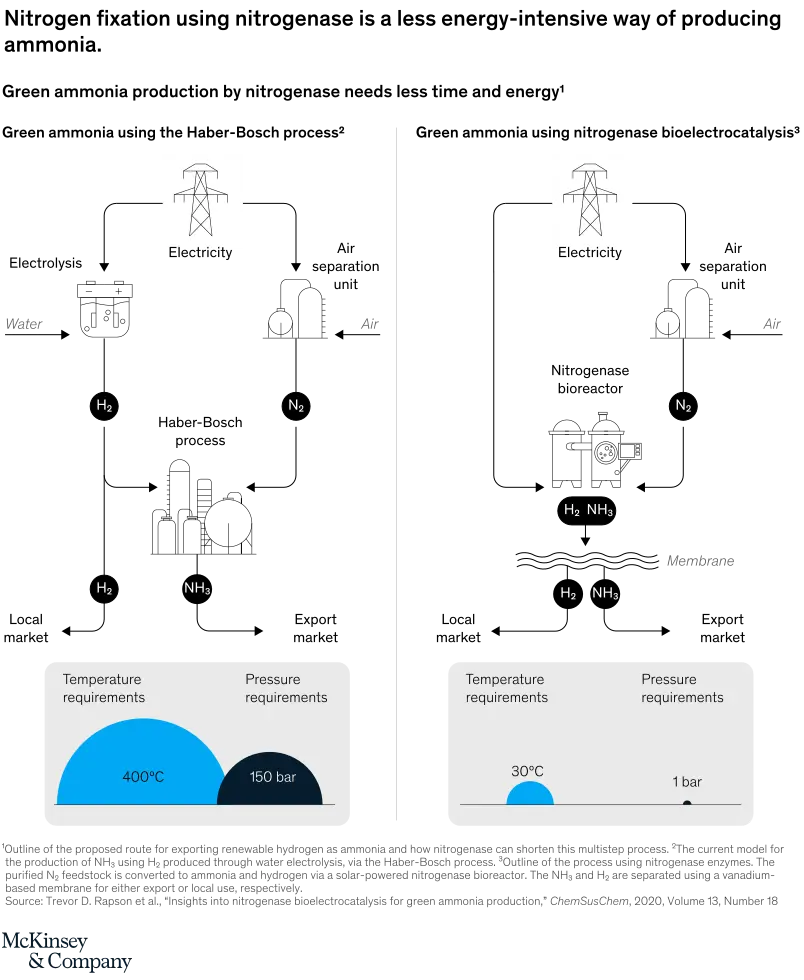
Currently ammonia is made through the energy-intensive Haber-Bosch process using natural gas. A tiny anaerobic bacteria in the roots of plants performs this same process every day at very low energy cost using a specific molecule—nitrogenase.
The MoFe protein, and the FeMoco, can be analyzed by quantum computing to help reveal the complex chemical system behind nitrogen fixation by the enzyme nitorgenase.